SPECIAL ORDER
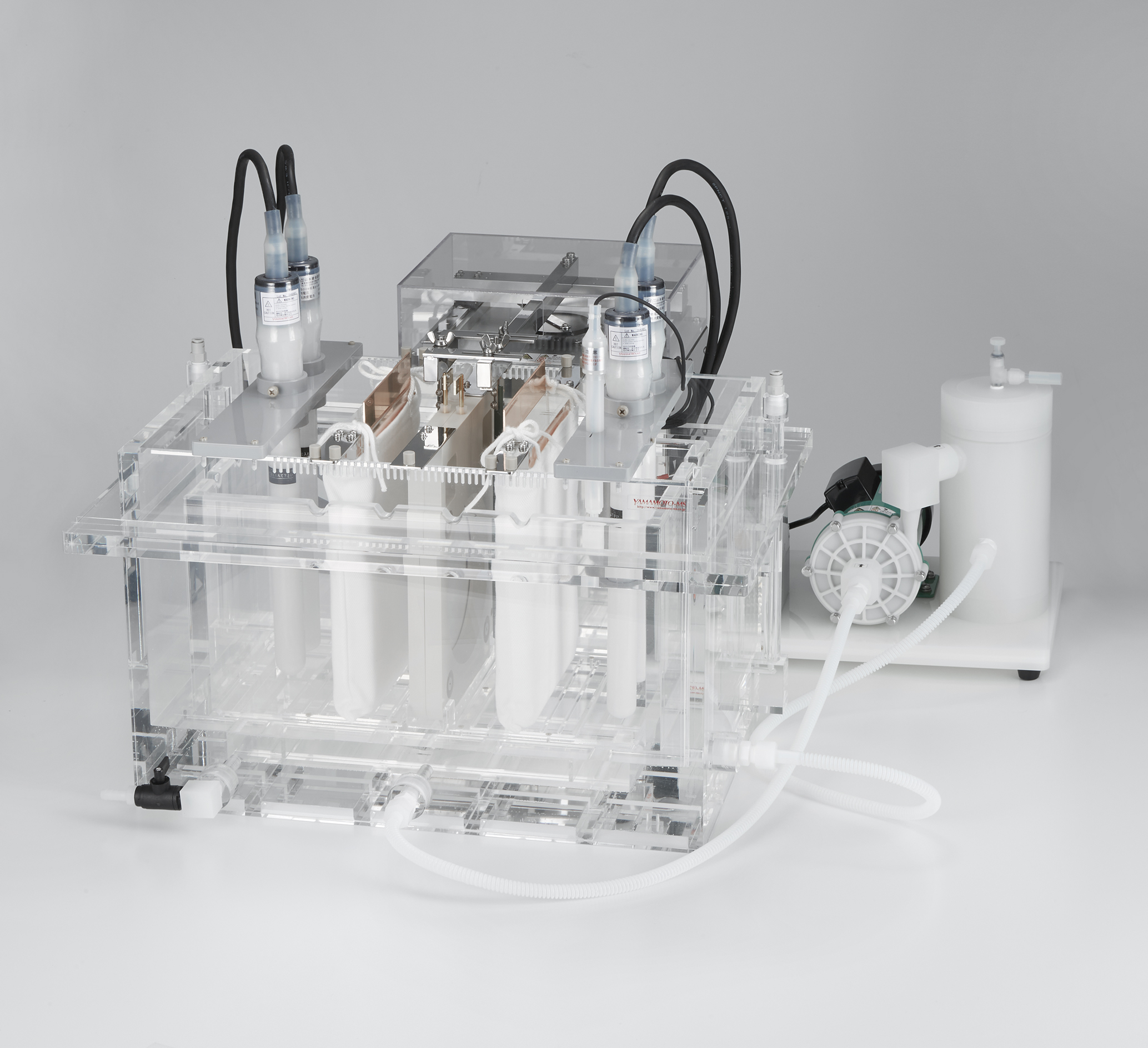
JET PLATING EQUIPMENT

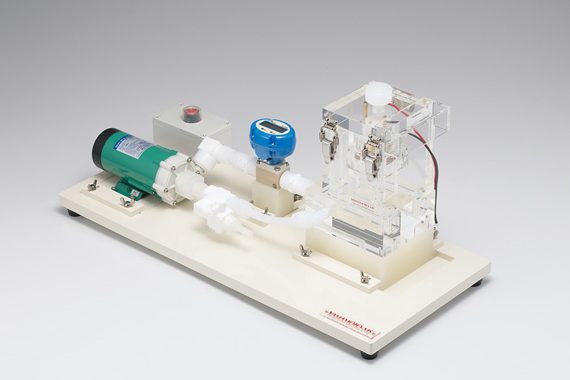
This kit makes it possible to perform a high speed plating test with a very high current density by applying high speed blowing of the solution. Jet plating experiments can be performed with as little as 500mL of liquid. The test piece
can be attach- and detachable, and can be affixed using uniform pressure. This kit is a semi-custom order product. As a built-to-order product, it is possible to design the kit for 1cm square to φ 4inch sized samples.
For details,please contact us.

Video of Jet Plating Equipment Experiment
The Reason why Jet Plating is effective for high-speed plating
In electroplating, the thickness of the deposited film usually increases proportionally to the quantity of electricity.
Plating thickness is basically proportional to current density i. Therefore, if the plating time is the same, the film will become thicker as the current density increases. However, if the current density becomes too high, a hydrogen reduction reaction will occur, resulting in the significant lowering of the current efficiency. When considering the mass balance of the electrode surface, the amount of metal deposited (Ne) within unit-time and over unit-area can be expressed according to Faraday’s Laws as below:
Ne=i/nF ……. ( 1 )
n :atomic valence of electrodeposited metal F :Faraday’s constant
Since metal ions of Ne amount are consumed on the electrode surface, this results in the lowering of the metal ion concentration near the electrode, forming a concentration gradient.

When a concentration gradient is caused, metal ions will be transported from the bulk of the solution to the plating surface by means such as diffusion, migration or convention. The amount that is transported (Nd) can be expressed using the formula below:
Nd =D(Co-C)/d ……. ( 2 )
D : diffusion constant of metal ion(dependant on plating solution temperature)
Co : metal ion concentration at bulk
C : metal ion concentration at the electrode surface
d : diffusion layer thickness
Deposited metal ions are supplied in bulk under a stabilized state. Therefore, Ne (deposited volume) will be equivalent to Nd (transported volume) Accordingly, ( 1 ) and ( 2 ) together can be expressed as:
i =nFD(Co-C)/d ……. ( 3 )
When the deposited volume > the transported volume, the metal ion concentration C at the electrode surface will decrease. If there are no more metal ions left, plating will no longer be deposited. The current under such situation is called the limiting current density (il), and metal ions at the interfacial surface become zero. Therefore, C=0 and from ( 3 ), the following formula can be expressed:
il =nFDCo/d ……. ( 4 )
If the current density should exceed the limiting current density, a substantial hydrogen reduction reaction will occur. In such cases, the resulting deposits will be rough and granular, eg. burnt deposits. In a Hull Cell test, such roughness tends to appear on the left side of the test piece (side closest to the anode). This is because, as shown in p.16, the current concentrates to the left area, exceeding the limiting current density. In general, there are two ways to prevent burnt deposits: (i)to include additives in the plating bath or(ii)to set a high limiting current density. High limiting current density can be achieved by setting a higher Co (metal ion concentration), a larger diffusion constant D (higher solution temperature), and a smaller diffusion layer thickness d (stronger agitation) in Formula( 4 ). Limiting current density will increase by performing all conditions or even just one of the aforementioned methods.Therefore, by performing only a fast spray of the solution, it is possible to carry out the plating of the high current density. However, in actual fact, the bath-balance cannot be maintained for some solutions if the metal ion concentration or solution temperature is set too high. Also, with some solutions, the effect of additives weakens if it is agitated too strongly.
This Jet Plating Laboratory Kit can be used in order to confirm such a condition. There is“HCHS A-45” in the same kind of test equipment for high- speed plating. Whereas the test piece of Jet Plating Kit is fixed and the diffusion thickness is varied by spraying solution on it, the test piece of HCHS is rotated at a high speed. Please make good use of it together.
Related Products
-

Plating Training Equipment
We can manufacture in-house plating training equipment and equipment which takes various skill tests into account.
-

Plating equipment with preparation tank and Electrolytic Refining equipment (A-64)
Plating equipment with pre-tank for electroforming, composite plating, barrel plating, wafer plating, electroless plating, anodic oxidation, electrolytic polishing, etc. on small parts.
-

C-BIKA-01 Biofilm reactor